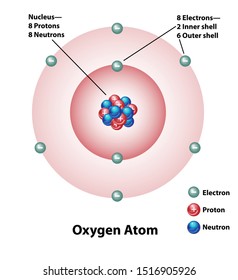
The Bohr model for oxygen shows eight protons and neutrons in the nucleus of the atom, with eight electrons orbiting the nucleus in two energy levelsIn the center, with six neutrons and sixFor illustration (I felt a little uneasy about that part);

Shell Model Of Carbon Dioxide
Model of carbon atom project
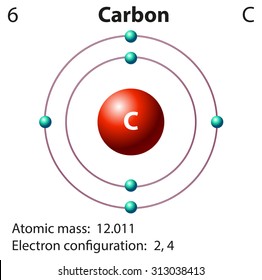
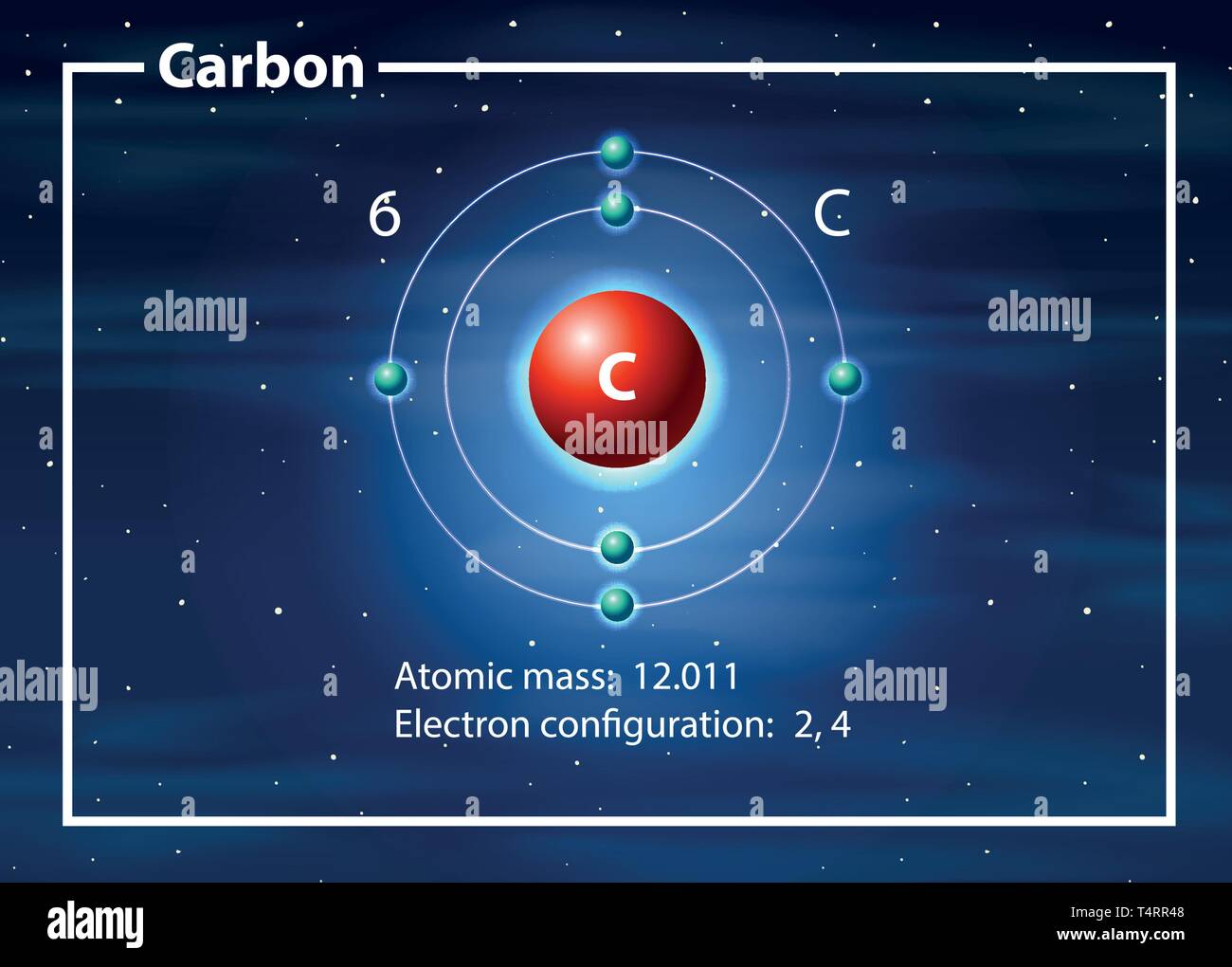
Model of carbon atom project-Carbon Dioxide This is a carbon dioxide molecule When you breathe out, you usually breathe out carbon dioxide With the formula CO 2 that means there are two oxygen (O) atoms and one carbon atom If you look closely at the dot structure, you'll see that they share four electrons eachMar 25, · The Bohr model of carbon has a central nucleus containing six protons and six neutrons, encircled by an inner orbit of two electrons and an outer orbit of four electrons The two orbits represent different energy levels and are at a set distance from one another and from the nucleus In the Bohr model, electrons with less energy occupy orbits




Carbon Atom On White Background Stock Photo Picture And Royalty Free Image Image
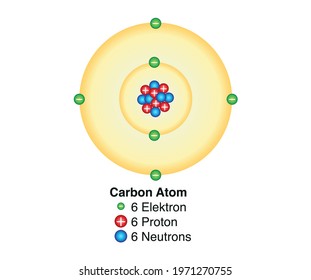
8th Grade Science Project Carbon Atom ModelBuild photos at http//bitly/carbonatomJul 21, 16 · Carbon is the sixthmost common element in the universe Carbon atoms contain 6 neurons, 6 protons, and 6 electrons We decided that creating a 3D carbon atom model was the perfect way to test out the new 3D pen from IDO3D We had to figure out how to make the individual carbon atoms and the circle for the electrons to go around the nucleusNov 21, · Carbon is a chemical element with atomic number 6 which means there are 6 protons and 6 electrons in the atomic structure The chemical symbol for Carbon is C The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons The nucleus is composed of protons and neutrons
Mar 31, 21 · 37,430 carbon atom stock photos, vectors, and illustrations are available royaltyfree See carbon atom stock video clips of 375 element carbon atomic structure carbon atomic structure vector atoms the atom carbon molecule atom diagram electron atomo an atom protons neutrons electrons carbon atomic modelApr 25, · Adding the two neutrons changes our atom However, because the number of protons are the same, it is still carbon but now it is an isotope of carbon What is the Bohr model for oxygen?Carbon (from Latin carbo "coal") is a chemical element with the symbol C and atomic number 6 It is nonmetallic and tetravalent —making four electrons available to form covalent chemical bonds It belongs to group 14 of the periodic table Carbon makes up only about 0025 percent of
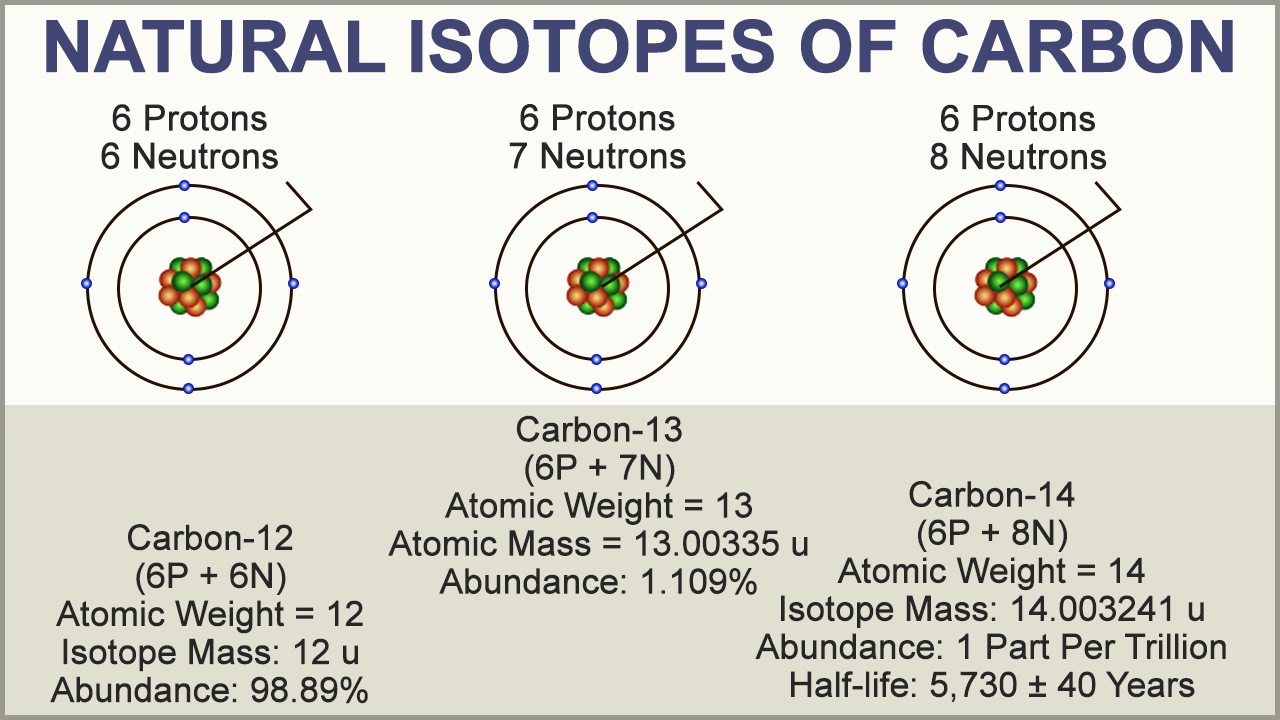
Different atoms of the same element with different weights are called isotopes For example, 12 C, 13 C and 14 C are all isotopes of carbon They all have six protons, but different numbers of neutrons, as seen in a model of 12C and 13C, below Neutrons are also in the nucleusApr 9, 14 Most students learn about atoms and characteristics of the elements on the periodic table in middle and high school science classes Consider choosing a simple atom, such as carbon, to represent through a hanging mobile 3D model Although simple in structure, carbon and compounds containing carbon form the basis of_____11 Unlike the modern model of the atom, Bohr's model states that a electrons move in set paths around the nucleus of an atom b atoms cannot be divided into smaller parts c electrons behave like waves d electrons contain orbitals _____12 According to the modern model of the atom, a moving electrons form an electron cloud b



The History Of The Atom Carbon Atom Png Free Transparent Clipart Clipartkey



Carbon Atom Model High Res Stock Images Shutterstock
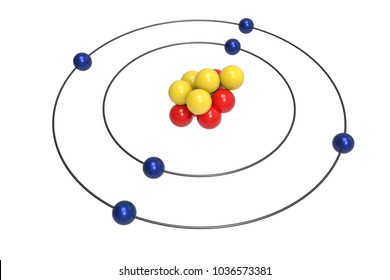
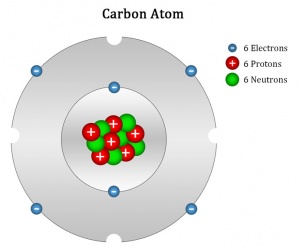
For carbon, the atomic number is 6, and the atomic mass number is 12 (6 protons plus 6 neutrons) Adding the two neutrons changes our atom However, because the number of protons are the same, it is still carbon but now it is an isotope of carbonThe valence shell electron pair repulsion (VSEPR) model _____ describes the threedimensional arrangement of the atoms in a molecule states that two negatively charged particles (electrons) will always repel one another with equal and opposite forces ensures bonding through shared valence electron pairsJan 07, 18 · The atomic number is the number of protons in an atom of that element So, you know you need 6 protons to make a model of carbon To make a carbon atom, make 6 protons, 6 neutrons, and 6 electrons Bundle the protons and neutrons together to make the nucleus and put the electrons outside the atom




Carbon Atomic Model Project Science Project Models Carbon Atom Model Atom Model



Atomic Structure Ms Smith S Class
Chemistry Model of Atom STUDY PLAY Atomic Models This is the Bohr model In this model, the nucleus is orbited by electrons, which are in different energy levels A model uses familiar ideas to explain unfamiliar facts observed in nature A model can be changed as new information is collected Democritus' "atomos"Twodimensional hexagonal crystal lattice formed by a layer of atoms of carbon in the thickness in one atom Atom and electrons Illustration of an atom with nucleus and six electrons around it Carbon dioxide, a colorless and odorless gas vital to life on Ea Rth present in deposits of petroleum and natural gasNov 02, 15 · What we ended up modeling was the carbon atom using the sp^3 hybrid orbitals depicted with four balloons as lobes in a tetrahedral configuration The inner 1s shell was depicted with another sphere, in the middle, with an oversized nucleus;



Carbon Atom Model High Res Stock Images Shutterstock



Sample 6 Chemistry Carbon Atom Accessible Image Sample Book
May 09, 12 · Sep 6, 15 Explore Martha McDowell's board "Atom model ideas" on See more ideas about atom model, atom, atom model projectA competing model had been proposed in 1903 by Hantaro Nagaoka, who postulated a Saturnlike atom, consisting of a positively charged sphere surrounded by a halo of electrons Figure \(\PageIndex{5}\) (a) Thomson suggested that atoms resembled plum pudding, an English dessert consisting of moist cake with embedded raisins ("plums")Browse 295 Carbon Atom stock photos and images available, or search for carbon molecule or atoms to find more great stock photos and pictures Molecular Model Colorful 3D Illustration of an atom, that is the smallest constituent unit of ordinary matter that has the properties of a
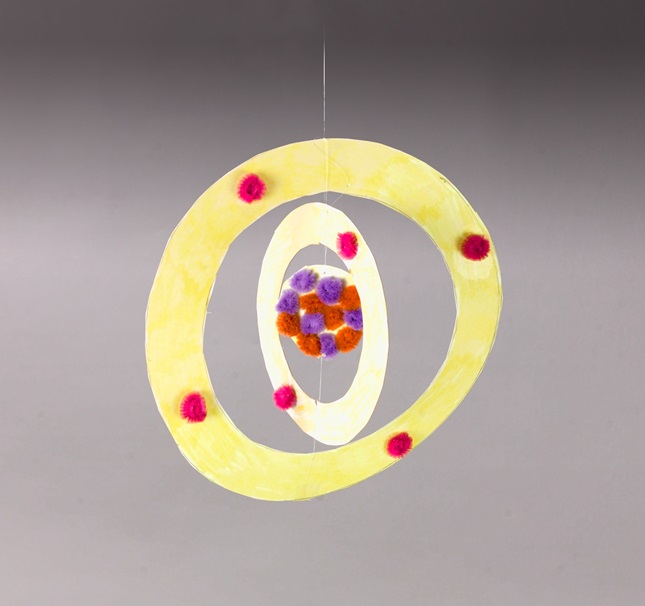



Carbon Atom Mobile Crayola Com
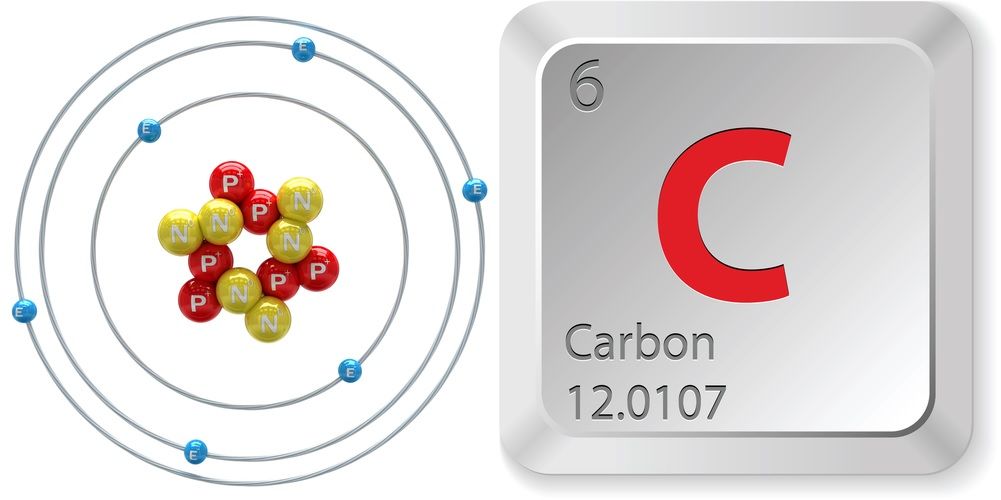



Carbon Element Facts Discovery Atomic Structure Uses Live Science
Nov 21, · An atom of Carbon in the gas phase, for example, gives off energy when it gains an electron toNov 07, 13 · 3D Model of Carbon Atom I used glow in the dark necklaces, clay, and string to make the model The glow in the dark necklaces represent the orbitals There are two orbitals The reason we know this is because it is in the second row on the Periodic Table of Elements With the clay, I used yellow to represent the electronsSearch from 3d Model Of Carbon Atom Pic stock photos, pictures and royaltyfree images from iStock Find highquality stock photos that you won't find anywhere else




A T O M I C S T R U C T U R E O F C A R B O N A T O M Zonealarm Results



Biochemistry Alma D Arte
Apr 24, 17 · Consider choosing a simple atom, such as carbon, to represent through a hanging mobile 3D model Although simple in structure, carbon and compounds containing carbon form the basis of all life Making a 3D model of a carbon atom can help students demonstrate their understanding of protons, neutrons and electrons that form atomic structureQ This model was developed after JJ Thompson discovered electrons, a particle smaller than an atom Is shows electrons floating freely in a positive spacePlace one orange ball onto the tip of a toothpick for the helium atom model Push the other end of the toothpick into the top of one of the other balls of clay This will represent the electrons within the atom's electron cloud A model of hydrogen (1), for example, would consist of zero red balls, one green ball, and one blue ball




3d Model Of Carbon Atom Stlfinder
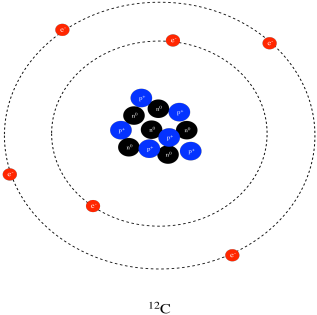



Structure Reactivity Atoms Protons Neutrons Electrons
CARBON 3D CHEMISTRY OF CARBON USING CHIME Compounds made of carbon, the wire frame model, the stick model, the ballandstick model, the space filled model of molecules CARBON 3D CHEMISTRY OF CARBON USING JMOL Note Jmol will run faster than Jsmol but requires access to Java to run CARBON 3D CHEMISTRY OF CARBON USING JSMOL JsmolThe radius of an atom is about 01 nm (1 × 1010 m) The structure of a carbon atom, not drawn to scale The masses of subatomic particles are very tinySep 24, 15 · Current model of atomic structure The current model of the structure of an atom is as follows The nucleus contains protons and neutrons, which are located at the center of the atom Electrons orbit the nucleus in shells, which contain the set of orbitals The nucleus is small, dense mass at the centre of the atom




Carbon Atom 3d Cad Model Library Grabcad
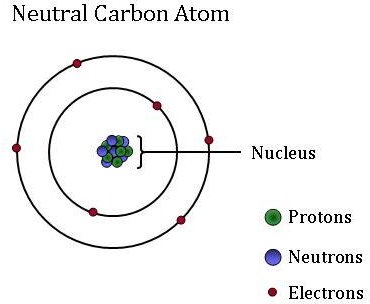



Global Monitoring Laboratory Carbon Cycle Greenhouse Gases
Its diameter is about 1012 cm, about 1/10,000 that of the whole atom Given this model, if the nucleus were the size of a marble, the atom with its extranuclear electrons would be 300 m in diameter If a marble had the same density as the nucleus of an atomJun 23, 16 · The nucleus is the small, dense region at the center of an atom where protons are also found Atoms generally have about the same number of neutrons as protons For example, all carbon atoms have six protons and most also have six neutrons A model of a carbon atom is shown in the figure belowJun 24, 15 · Step 1 You will need 12 large balls (6 of one colour for the protons and 6 of another colour for the neutrons) and 6 small balls for the electrons Glue the six protons and six neutrons into a ball, alternating between protons and neutrons as you glue Step 2 Cut a small ring and a large ring out of cardstock




Worldofchemicals Atomic Theory Atom Diagram Atom Model



Illustrated Glossary Of Organic Chemistry Carbon 13 13c
Most students learn about atoms and characteristics of the elements on the periodic table in middle and high school science classes Consider choosing a simple atom, such as carbon, to represent through a hanging mobile 3D model Although simple in structure, carbon and compounds containing carbon form the basis of all life Making a 3D model of aA better way to look at the carbon atom is by using an energy level graph shown at the right Here we see carbon has six electrons represented by arrows (the direction of the arrow represents the electron spin) Two electrons are found in the 1s orbital close to the nucleusIn this model, each electron energy level is denoted by numbers that take concentric shells as suggested by the Bohr model because there are overlaps in the ordering of the energy levels In the case of the carbon atom the electrons occupy four tear drop shaped clouds in a




Carbon Atomic Structure Stock Image C018 3687 Science Photo Library




Atom Definition Structure History Examples Diagram Facts Britannica
Adding or subtracting neutrons from the nucleus of an atom creates isotopes of that atom For instance, lets add two neutrons to the carbon atom, represented by green dots below Carbon isotope Adding the two neutrons changes our atom However, because the number of protons are the same, it is still carbon but now it is an isotope of carbonThis is the quantum model of carbon atom In the spaces given by orbitals, you have 90% probability of finding electron At all levels the electron cloud covers theAug 26, 17 · The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei If the atom is neutral, it will have the same number of negatively charged electrons Its electron configuration is 1s22s22p2 The orbital diagram shows how the electrons are arranged within each sublevel




Carbon Atom




Download Hd Transparent Atom Carbon Transparent Png Image Nicepng Com
The heart of carbon chemistry is, of course, the carbon atom Like all atoms, the carbon atom is made of only three particles protons, neutrons, and electrons There are several ways to represent a carbon atom Each model has strengths and weaknesses This is called the electron cloud model It shows how the carbon atom looks under an electronJan 13, 13 · Example Carbon12 For example, the most common form (isotope) of carbon12 is written as Protons = 6 Since we know the atomic number is 6 (because we memorized it), the atom has 6 protons Neutrons = 6 Since the atomic mass is 12 (upper left of the element symbol), to find the number of neutrons we subtract the number of protons (12 – 6 = 6)Aug 30, 14 · It is interesting to note the carbon atom has 6 electrons, 6 protons and 6 neutrons The graphic represents a model for the carbon atom This is the base atomic structure of our elemental body on the earth Protons, electrons and neutrons build elements in a straight forward manner For each additional proton, a new element is created




Carbon Atom 3d Model Using Candy And Pancakes Science For Kids Science Projects School Projects




How Many Neutrons Does Carbon 14 Have Learn Lif Co Id
Atomic carbon, systematically named carbon and λ 0methane, also called monocarbon, is colourless gaseous inorganic chemical with the chemical formula C (also written C) It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation Atomic carbon is the simplest form of carbon, and is also the progenitor of carbon clustersMay 06, 19 · Key Takeaways Model of the Atom An atom is a building block of matter that cannot be broken apart using any chemical means Nuclear reactions can alter atoms The three parts of the atom are protons (positively charged), neutrons (neutral charge), and electrons (negatively charged) Protons and neutrons form the atomic nucleusQuantum Mechanical Model of Atom Quantum mechanics is based on Schrödinger's wave equation and its solution The solution of the wave equation brings the idea of shells, subshells and orbitals The probability of finding an electron at a point within an atom is proportional to the ψ 2 at that point, where ψ represents the wavefunction
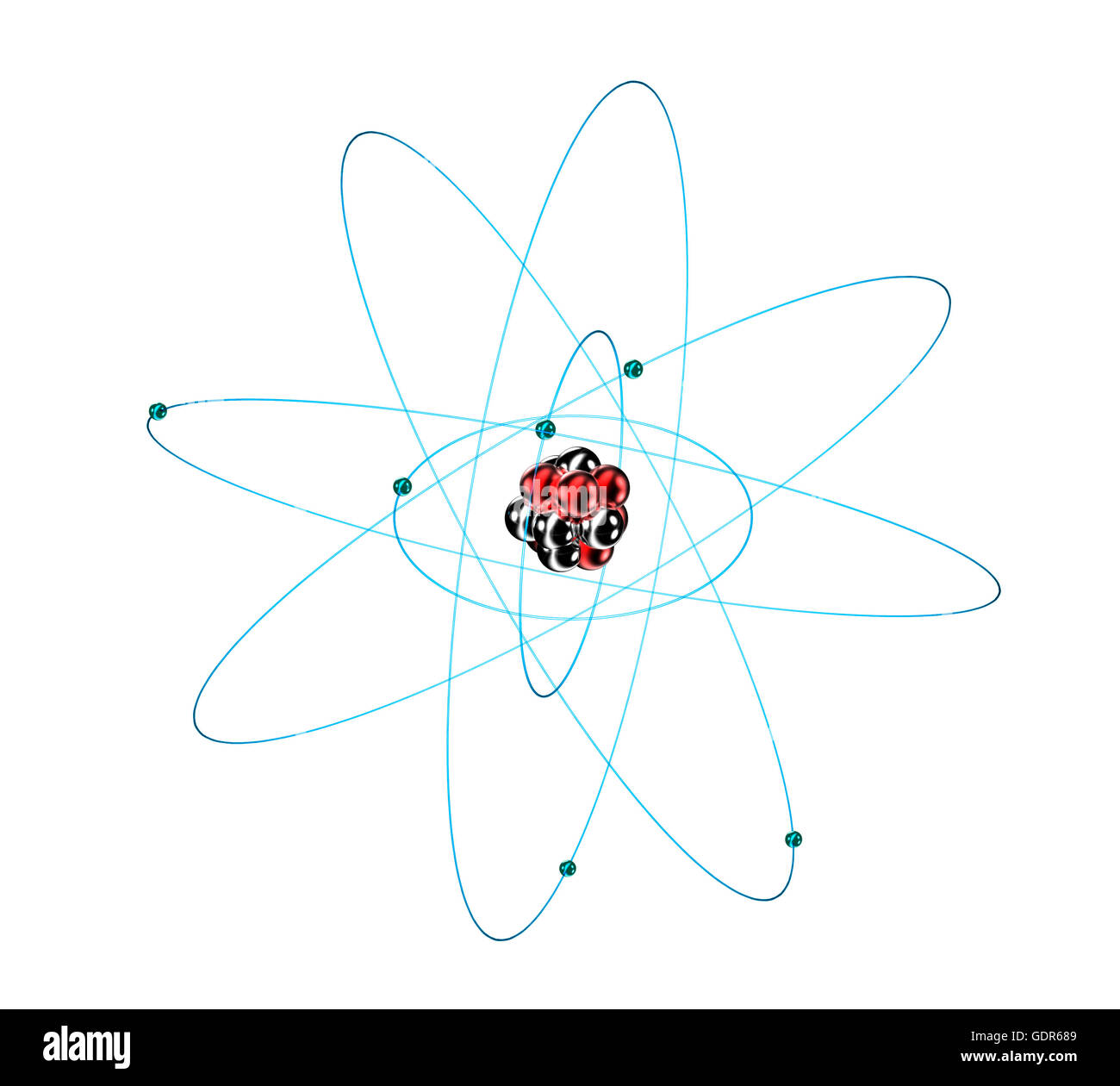



Carbon Atom Cut Out Stock Images Pictures Alamy




Bohr Model Description Development Britannica




Carbon Atom 3d Model 29 Unknown Ige Sldpr Free3d
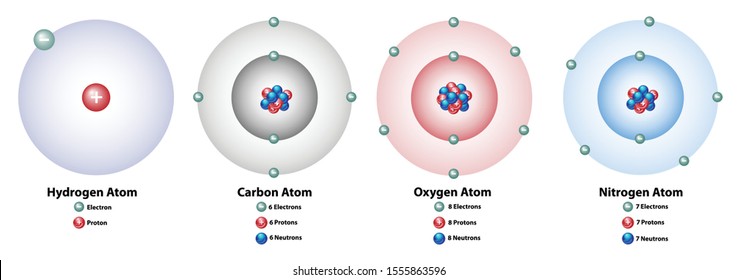



Carbon Atom High Res Stock Images Shutterstock




What Is Carbon Facts History Lesson For Kids Study Com




File Carbon Atom Jpg Wikimedia Commons



The Carbon Atom Book Chapter Iopscience
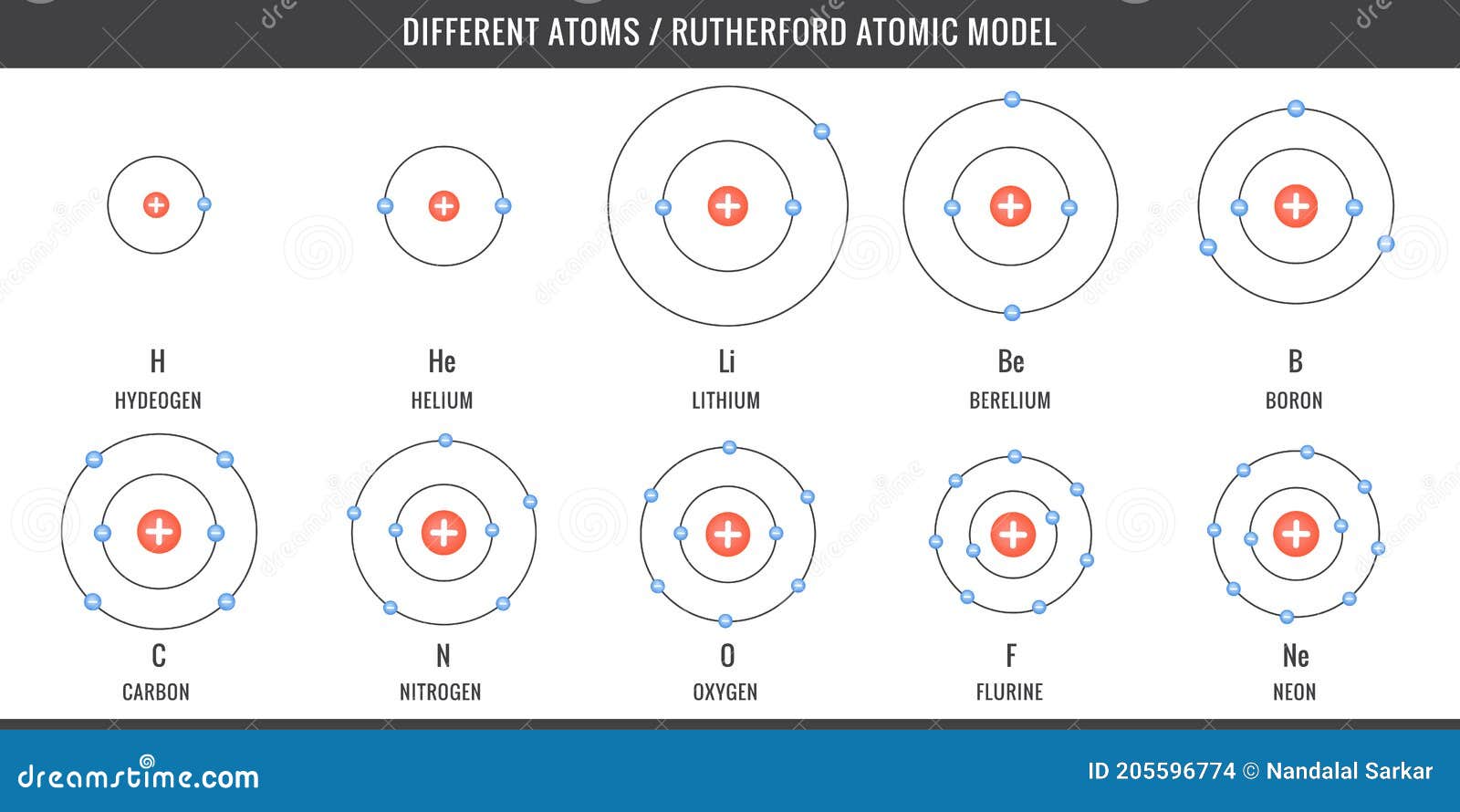



Different Atoms Simplest Atomic Model Of Hydrogen Carbon Oxygen Nitrogen Stock Vector Illustration Of Diagram Atom




Paper Plate Model Of Carbon Ppt Download




Rutherford Model Of The Atom Definition Nuclear Power Net




What Is An Atom It S A Question Of Physics The Atomic Age Linda Hall Library Kansas City Mo
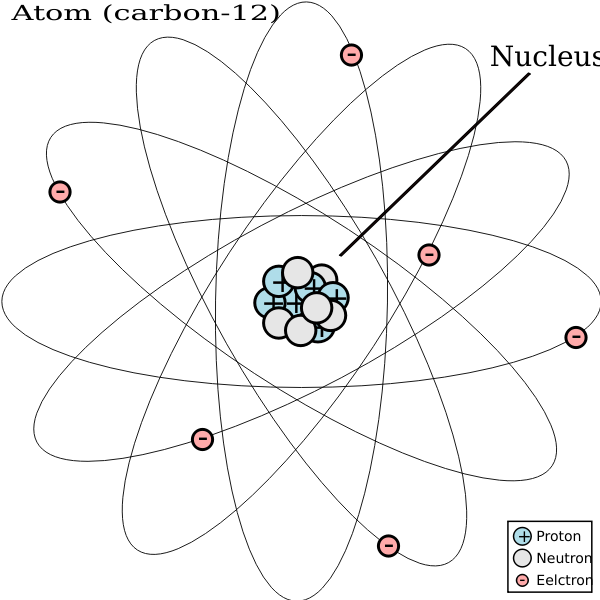



Carbon 12 Atom Diagram Vector Image Free Svg
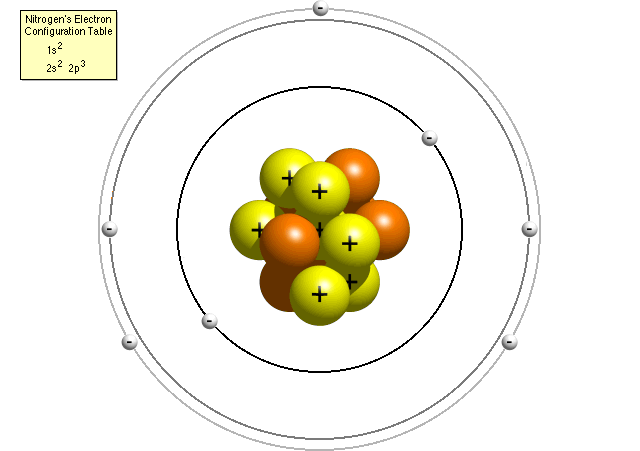



Questions And Answers How Do I Make A Model Of An Atom




A Science Odyssey Atom Builder Building An Atom
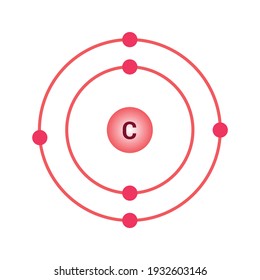



Carbon Atom Model High Res Stock Images Shutterstock



Carbon Atom Carbon Atom Free Transparent Png Clipart Images Download
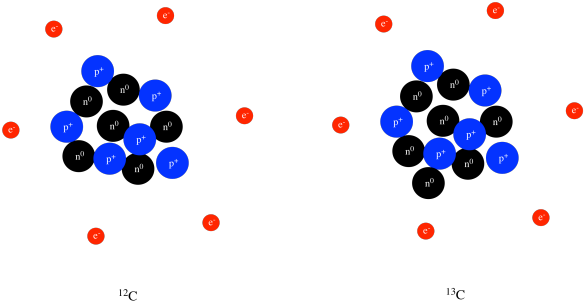



Structure Reactivity Atoms Protons Neutrons Electrons




Shell Model Of Carbon Dioxide



According To The Quantum Mechanical Model Of An Atom How Can I Imagine A Carbon Atom Quora



Free Photo Carbon Science Organic Chemistry Atoms Atom Max Pixel



Drawing Atoms Montessori Muddle




A New Model Of The Atom Wikibooks Open Books For An Open World




Carbon Atom 6th Grade Project Atom Model Project Chemistry Projects Science Project Models




Make A 3d Carbon Atom Model For Science




Carbon Atom Model Youtube
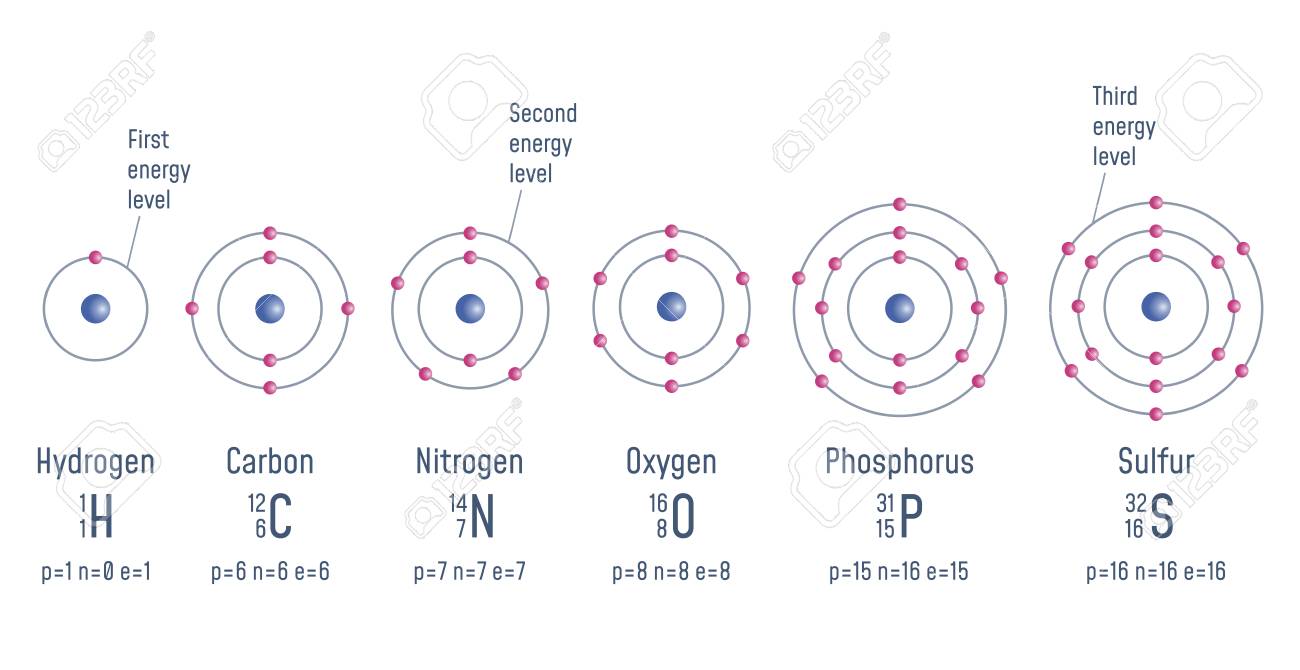



Structure Of An Atom Hydrogen Carbon Nitrogen Oxigen Phosphorus Stock Photo Picture And Royalty Free Image Image




What Is An Atom It S A Question Of Physics The Atomic Age Linda Hall Library Kansas City Mo




Carbon 12 Atomic Structure Stock Image C047 5347 Science Photo Library
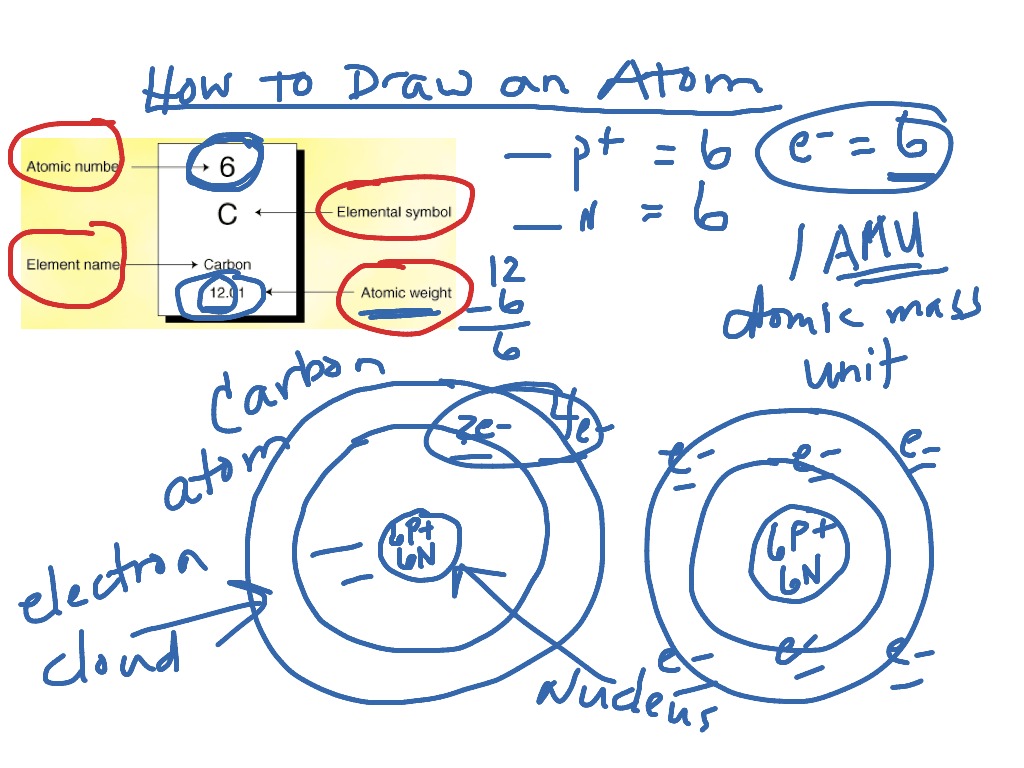



How To Draw An Atom Of Carbon Science Showme
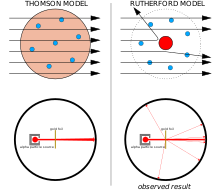



Atom Wikipedia




Carbon Atom 3d Cad Model Library Grabcad




Carbon Atom On White Background Stock Photo Picture And Royalty Free Image Image




Quantum Model Of Carbon Youtube




10 Best Carbon Atom Ideas Atom Atom Model Atom Model Project




2 1 Elements And Atoms The Building Blocks Of Matter Anatomy Physiology
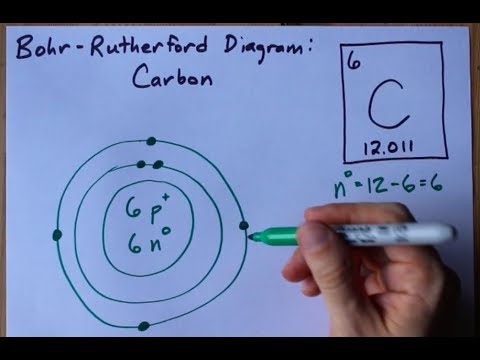



How To Draw The Bohr Rutherford Diagram Of Carbon Youtube
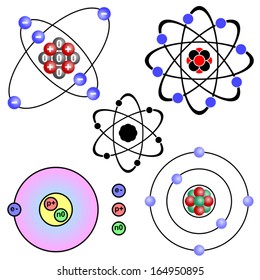



Carbon Atom Model High Res Stock Images Shutterstock



Climate Science Investigations South Florida Causes Of Climate Change
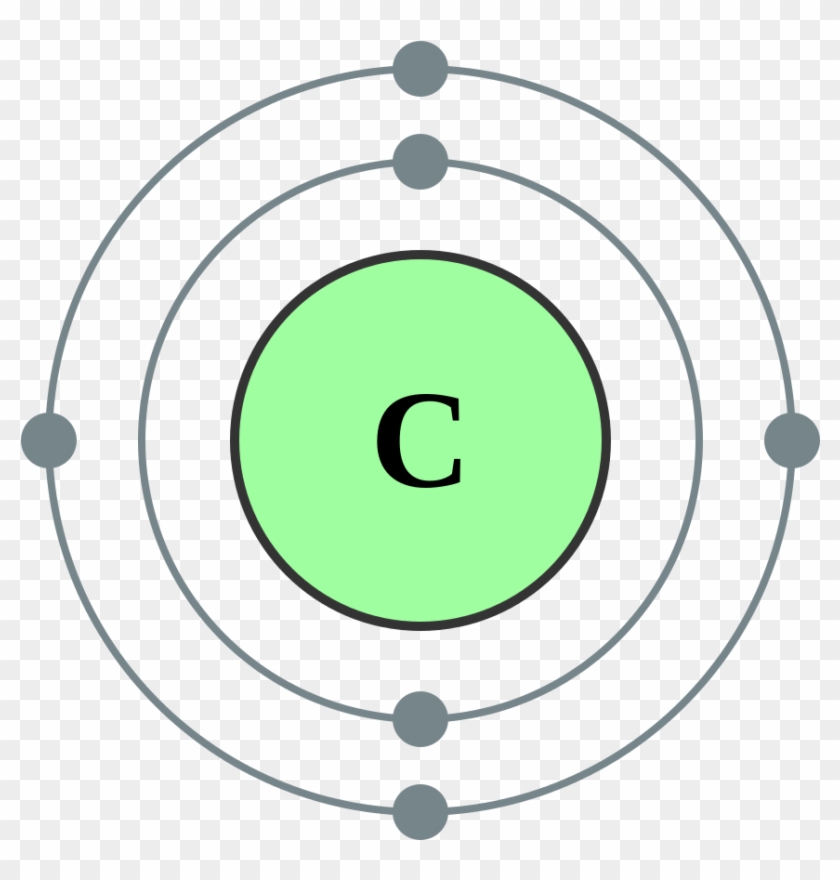



Boron Atom Model Carbon Electron Shell Diagram Hd Png Download 1000x1000 Pngfind
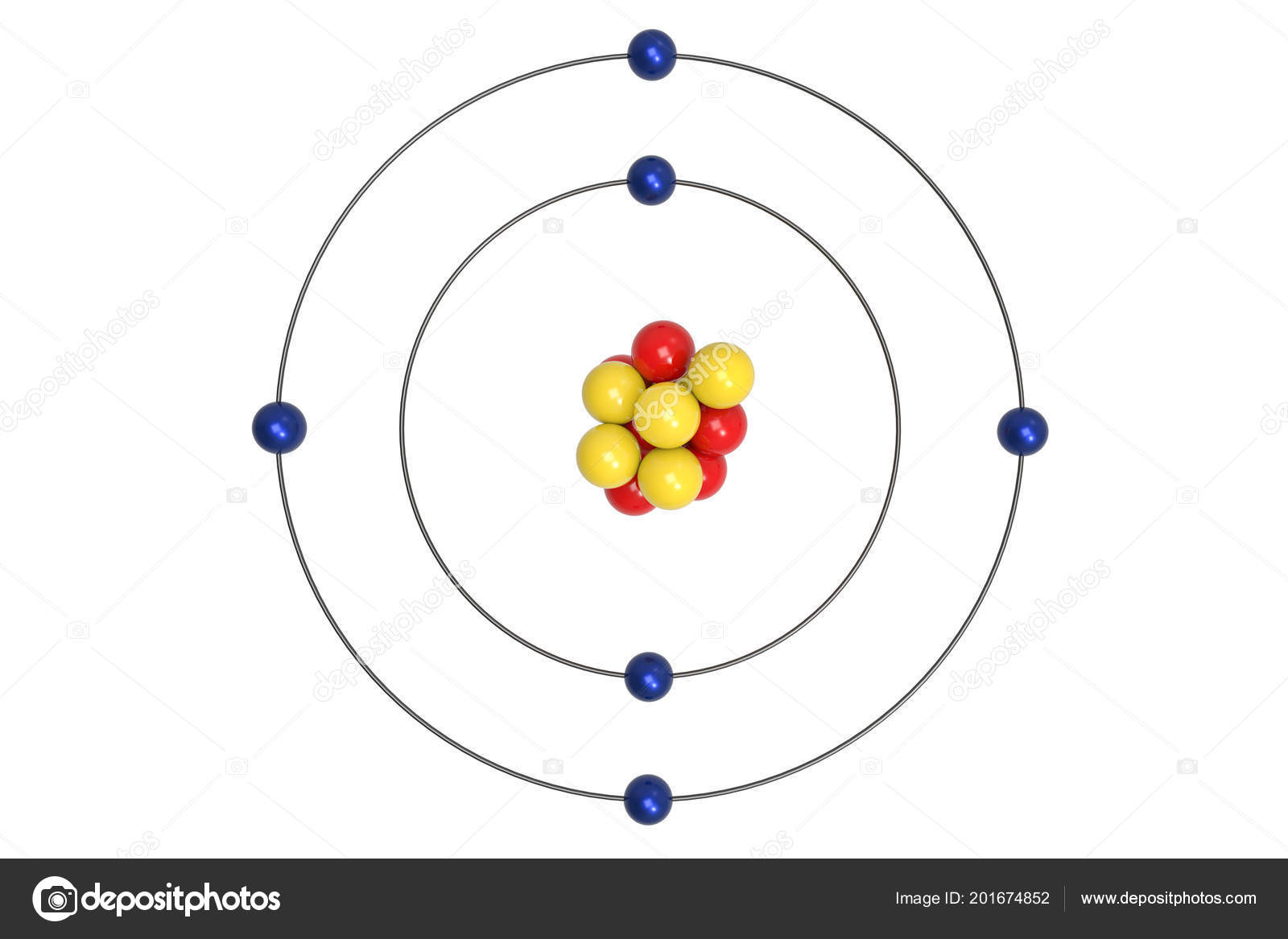



Carbon Atom Bohr Model Proton Neutron Electron Illustration Stock Photo Image By C Ema




Carbon Dioxide Atom Model Co2 Red Blue Illustration Stock Illustration Download Image Now Istock
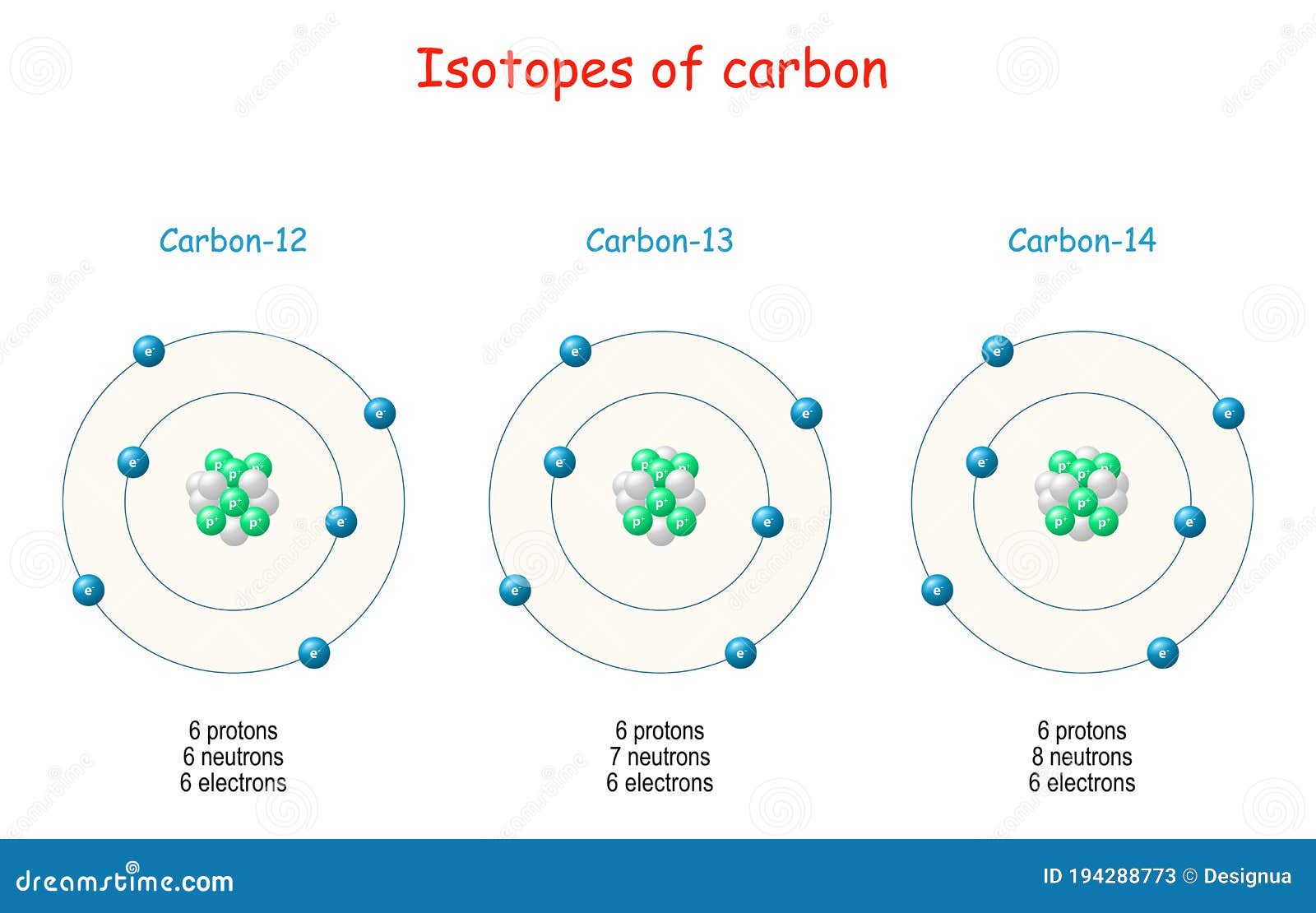



Carbon Isotope Stock Illustrations Carbon Isotope Stock Illustrations Vectors Clipart Dreamstime
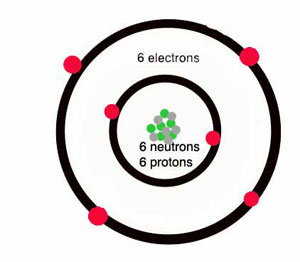



What Is Carbon Atoms Chemistry Quatr Us Study Guides



Atomic Structure



How To Make A 3d Model Of An Atom



Atomic Structure




2 1 Elements And Atoms The Building Blocks Of Matter Anatomy Physiology
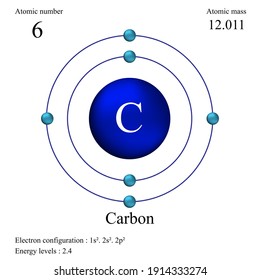



Carbon Atomic Model Hd Stock Images Shutterstock




Why Carbon 12 Youtube
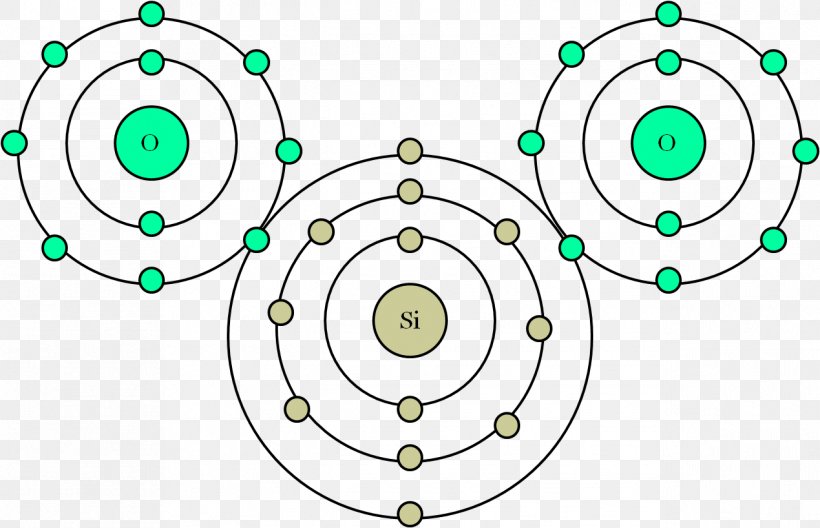



Bohr Model Atomic Theory Carbon Dioxide Png 1269x818px Bohr Model Area Atom Atomic Number Atomic Theory
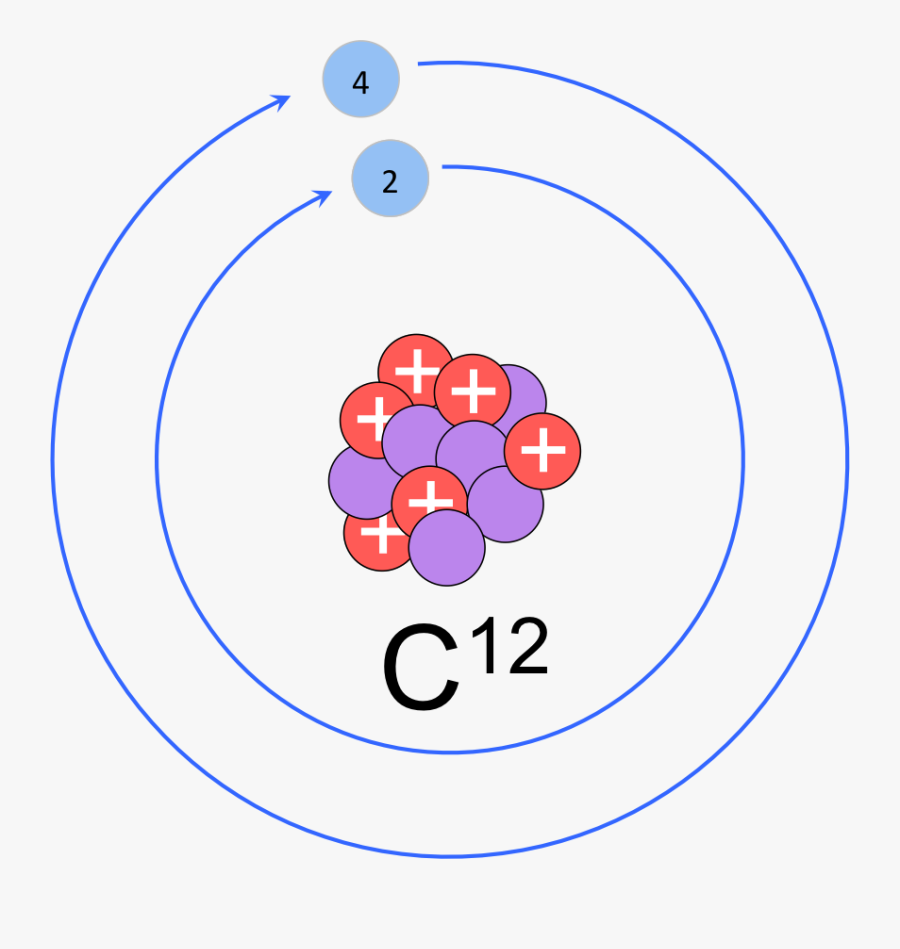



Clip Art Atoms Molecules E Chapter Model Of Carbon 12 Atom Free Transparent Clipart Clipartkey
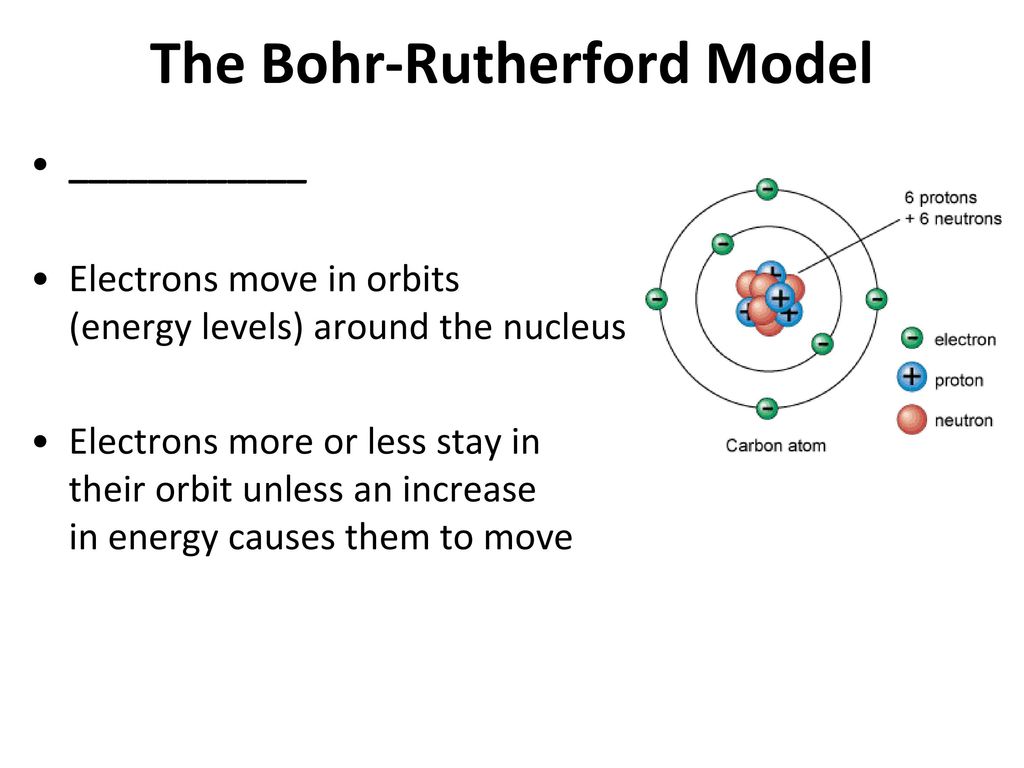



Atomic Theory And Structure Overview Ppt Download




Spiritual Secrets In The Carbon Atom Freemeditation Com



Transparent Atom Carbon Scientific Linux Logo Hd Png Download 1800x50 Pngfind




Rutherford Model Of The Atom Definition Nuclear Power Net



Classification




Carbon Atom An Overview Sciencedirect Topics




Carbon Atomic Structure High Resolution Stock Photography And Images Alamy



Reasons To Craft Your Own Molecular Models Ideas Rsc Education



Chem4kids Com Carbon Orbital And Bonding Info
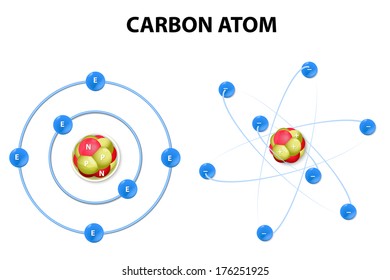



Carbon Atom Model High Res Stock Images Shutterstock




How To Make A 3d Model Of A Carbon Atom




Carbon Atom




Two Atom Model With Carbon Atoms Atom Model With Carbon Atoms Van Leest Antiques



Carbon Atom Model High Res Stock Images Shutterstock




Researchers Discover A Way To Tease Oxygen Molecules From Carbon Dioxide



Atomic Structure




Carbon 14 Atomic Structure Stock Image C047 5352 Science Photo Library




Model Of Coronene Molecule With Inner Carbon Rings And Outer Hydrogren Download Scientific Diagram



Carbon Atom Model High Res Stock Images Shutterstock




The Element Carbon Carbon Element Carbon Atom Model Atom Model




Carbon Atom An Overview Sciencedirect Topics



Carbon Atom High Resolution Stock Photography And Images Alamy



Carbon Atom Ascension Glossary




Carbon Atom 3d Model 29 Unknown Ige Sldpr Free3d



Carbon Atoms Atom Carbon Atom Png Free Transparent Clipart Clipartkey




Make A 3d Carbon Atom Model For Science


